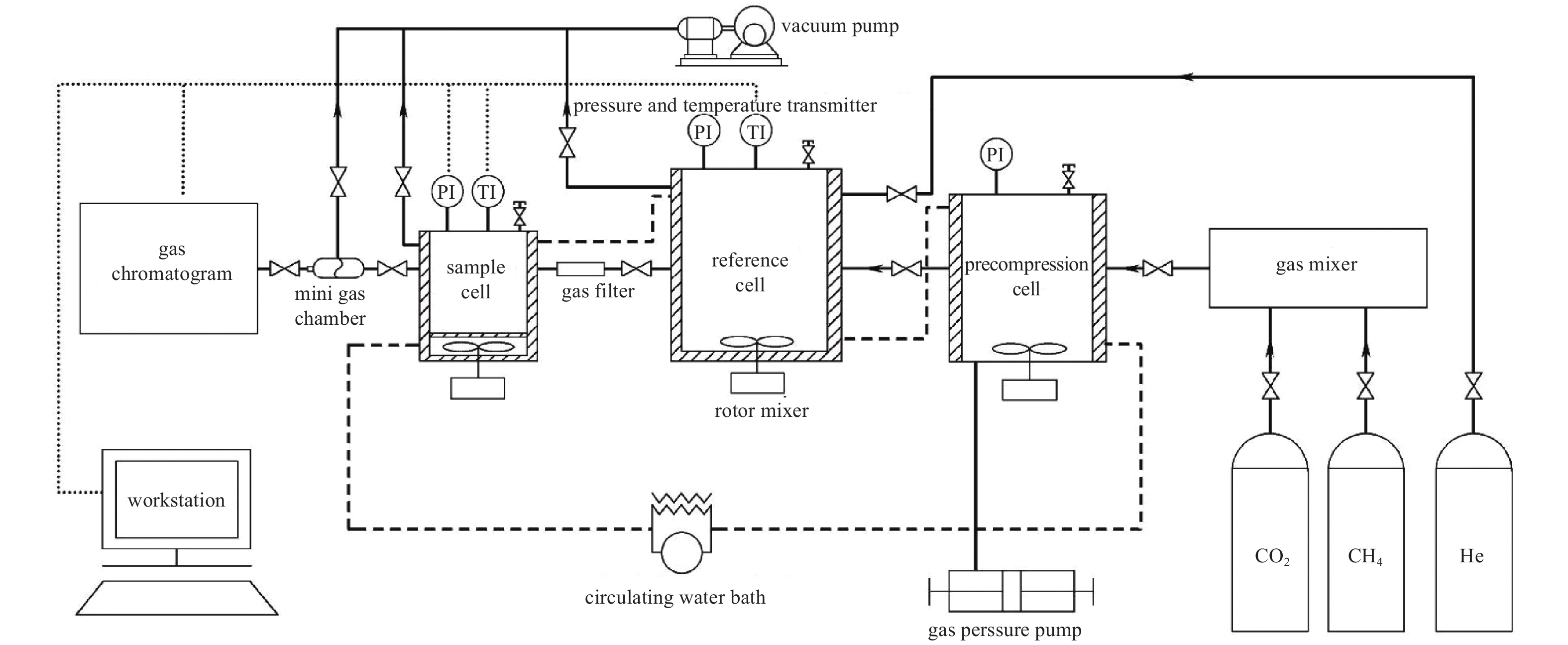
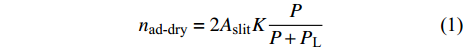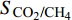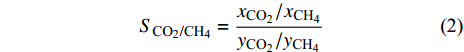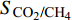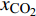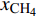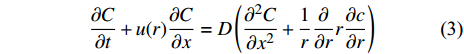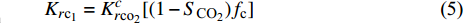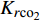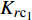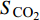REVIEW ON CO2/CH4 ADSORPTION AND DISPLACEMENT CHARACTERISTICS OF MICRO-NANO PORES IN SHALE RESERVOIR
-
摘要: 利用CO2开采页岩气不仅能够提高页岩气采收率, 还能够节省水资源并且对CO2进行地质封存, 有助于实现页岩气开采过程的碳中和. 富有机质页岩储层纳微米孔隙中气体运移机制不同于常规储层, CO2在储层中具有超临界特性, 致使开采机理复杂, 无法得到CO2开采页岩气微观机理的准确认识, 所以研究CH4, CO2及其二元混合物在页岩储层纳微米孔隙中的吸附及驱替特性对准确评估和高效开采页岩气至关重要. 本文从实验、理论以及模拟方面对页岩储层纳微米孔隙中CH4的吸附特性、CO2/CH4二元混合物竞争吸附特性以及驱替特性进行了综合分析, 对气体在纳微米孔隙中吸附及驱替特性的基础研究及关键问题进行讨论分析并提出了展望. 研究表明CH4在页岩储层中表现为物理吸附, 有机质特征(丰度、成熟度、类型)、孔隙结构、无机矿物组成、温度和压力、含水率对页岩的CH4吸附能力均有一定程度的影响. 在相同条件下, CO2比CH4更易被页岩储层吸附, 在页岩储层中注入CO2可以促进CH4的解吸, 并有利于CO2的地质埋存. 开采方案的部署可采用井网形式的注采方式, 可以通过调整注入井的位置、数量以及CO2注入速率对开采方案进行优化.Abstract: Using CO2 to produce shale gas can not only improve shale gas recovery, but also save water resources and carry out geological storage of CO2, which is helpful to achieve carbon neutrality in shale gas production process. The gas migration mechanism in micro-nano pores of organic-rich shale reservoir is different from that of conventional reservoir. CO2 has supercritical properties in the reservoir, which makes the exploitation mechanism complicated and it is impossible to obtain an accurate understanding of the microscopic mechanism of CO2 exploitation of shale gas. Therefore, it is very important to study the adsorption and displacement characteristics of CH4, CO2 and their binary mixtures in micro-nano pores of shale reservoir for accurate evaluation and efficient exploitation of shale gas. In this paper, review on the adsorption properties of CH4, CO2/CH4 binary mixture competitive adsorption and displacement properties in micro-nano pores of shale reservoir were carried out from three aspects: experiment, basic theory and numerical simulation. The key question and research trend on the CO2/CH4 adsorption and displacement characteristics of micro-nano pores in shale reservoir has been discussed. The results show that CH4 is physically adsorbed in shale reservoirs, and the characteristics of organic matter (abundance, maturity and type), pore structure, inorganic mineral composition, temperature and pressure, and water content all have a certain degree of influence on CH4 adsorption capacity of shale. Under the same conditions, CO2 is more easily adsorbed by shale reservoir than CH4. Injecting CO2 into shale reservoir can promote the desorption of CH4 and facilitate the geological storage of CO2. The deployment of the production plan can adopt the injection production method in the form of well pattern. The production plan can be optimized by adjusting the location, number and CO2 injection rate of injection wells.
-
Keywords:
- shale /
- micro-nano pore /
- CO2/CH4 /
- adsorption /
- displacement
-
引 言
随着全球能源需求的不断增加, 非常规油气资源的开发利用成为解决能源危机的有效途径[1-2]. 页岩气是一种重要的非常规油气资源, 其主要分布在中国、阿根廷、美国等地区, 中国页岩气资源分布广泛[3], 如图1, 并且经探明其可采资源量达36万亿立方米, 占全球页岩气可采资源量的20%, 居世界首位[4].
页岩气的成藏模式属于典型的“自生自储、原地成藏”, 其构成成份主要以甲烷为主(70% ~ 90%), 另外还含有少量其他轻质烃等. 页岩气赋存形态主要有两种: 一种是游离气, 通常赋存于基质裂缝和粒间孔隙中; 另一种是吸附气, 通常吸附在无机物或有机质表面, 其中吸附态气体比例占总气量的20% ~ 80%. 此外由于部分页岩储层含水, 所以有部分页岩气以溶解气赋存于水中[5, 6]. 页岩储层基质构成复杂, 含有丰富的有机质和不同比例的无机矿物, 主要包含有机物(干酪根)、黏土矿物(高岭石、伊利石等)、石英、方解石、黄铁矿等[7-9], 由于纳米孔的丰富, 其构成的孔隙结构复杂且跨度大, 一般可将页岩孔隙分为微孔(< 2 nm)、中孔(2 ~ 50 nm)、大孔(> 50 nm)3类[10-11]. 孔隙结构对页岩油气的赋存状态和储集能力有重要影响[12]. Zhang等[13]研究了页岩储层的孔隙特征, 发现95.6%的页岩孔隙是纳米级孔隙, 其中孔径在2 ~ 50 nm的中孔约占76%. 页岩气赋存形式以及页岩储层的复杂性造成了开采方式的特殊性, 页岩气开采通常采用水力压裂法[14-16], 在水力压裂过程中, 页岩气储层被泵入大量液体, 返排率通常较低. 这一过程不仅需要消耗大量水资源, 而且压裂液中的添加剂存在污染地下水和地表水的风险, 压裂也会造成页岩储层孔隙封闭、降低孔隙度等[16]; 与此同时, CO2的利用和地下封存技术被认为是缓解全球变暖的有效途径之一[17]. 通过研究表明, CO2能够封存于页岩基质中, 并且在页岩储层中注入CO2会对页岩气产生驱替作用, 从而提高页岩气采收率[18-19], CO2封存和提高天然气采收率(CS-EGR)技术的出现迅速得到研究人员的关注. 相比较于水力压裂法, CS-EGR技术有很多优势, 如CO2的封存不会破坏原有的地层结构、对页岩储层损害较小、可以提高页岩气的采收率、适用于更复杂的页岩储层等[19].
页岩储层基质构成及孔隙结构复杂, CH4和CO2在纳微米级孔隙中吸附及流动机制不同于常规储层, 所以本文主要针对页岩储层纳微米孔隙中CH4及CO2/CH4二元混合物的赋存状态、吸附特性, 分析了影响CH4在页岩孔隙中吸附的主要影响因素, 从实验、理论、数值模拟3个方面综述了CO2/CH4二元混合物在页岩储层纳微米孔隙中的竞争吸附特性及驱替特性, 对于利用CS-EGR技术高效开采页岩气发展趋势进行了展望, 具有重要的实际意义.
1. CH4吸附特性
页岩储层纳微米孔隙中的气体通常被认为是物理吸附, 尹帅等[20]对页岩中的CH4进行了等温吸附实验分析, 其实验结果表明CH4在该页岩上的吸附热大致在10 ~ 25 kJ/mol. 隋宏光和姚军[21]采用分子模拟的方法探究了CH4在干酪根壁面的吸附热, 研究结果表明CH4的最大吸附热为21.42 kJ/mol, 这些研究者均得到了CH4的吸附热小于42 kJ/mol的结果, 证明了CH4气体在页岩储层中属于物理吸附. 基于这一结论, 许多研究人员通过实验及分子模拟等方法进一步探究了页岩储层中影响CH4吸附的多种因素.
目前, 从宏观角度探讨页岩纳微米孔隙中CH4的吸附特性主要采用实验的方法, 并从页岩中有机质的特征、纳米孔隙结构、温度和压力等因素对页岩吸附CH4能力的影响进行研究[22-26]. 郭春礼等[27]对来自同一地区的修武盆地下寒武统海相页岩做CH4吸附实验, 发现影响CH4吸附的主要因素为有机质含量(TOC)和孔隙结构. 经过页岩微孔结构定量表征发现页岩中TOC含量越高, 会导致有机质中产生大量的中孔和微孔, 而这些微孔越多, 比表面积越大, 又因为大部分CH4分子吸附在微孔之中, 少部分分子单层吸附在中孔中, 所以气体吸附位点越多, 吸附能力就越强[28]. 不同地区页岩吸附能力也有差距, 沈伟军等[29]通过实验对比发现美国Woodford页岩的等量吸附热大于中国南方龙马溪页岩的等量吸附热, 这与页岩物理性质和成熟度有关, 页岩吸附热越大, 吸附越强. 陈磊等[30]对采自四川盆地上三叠统须家河组五段的页岩样品进行高压CH4等温吸附实验, 也得出了类似的结论, 即更不规则的孔隙表面与更复杂的孔隙结构会为CH4的吸附提供更多的空间, 使页岩的吸附能力更强. 朱汉卿等[31]通过实验也得出了TOC含量是影响蜀南地区龙马溪组海相页岩吸附甲烷能力的主要因素的结论.
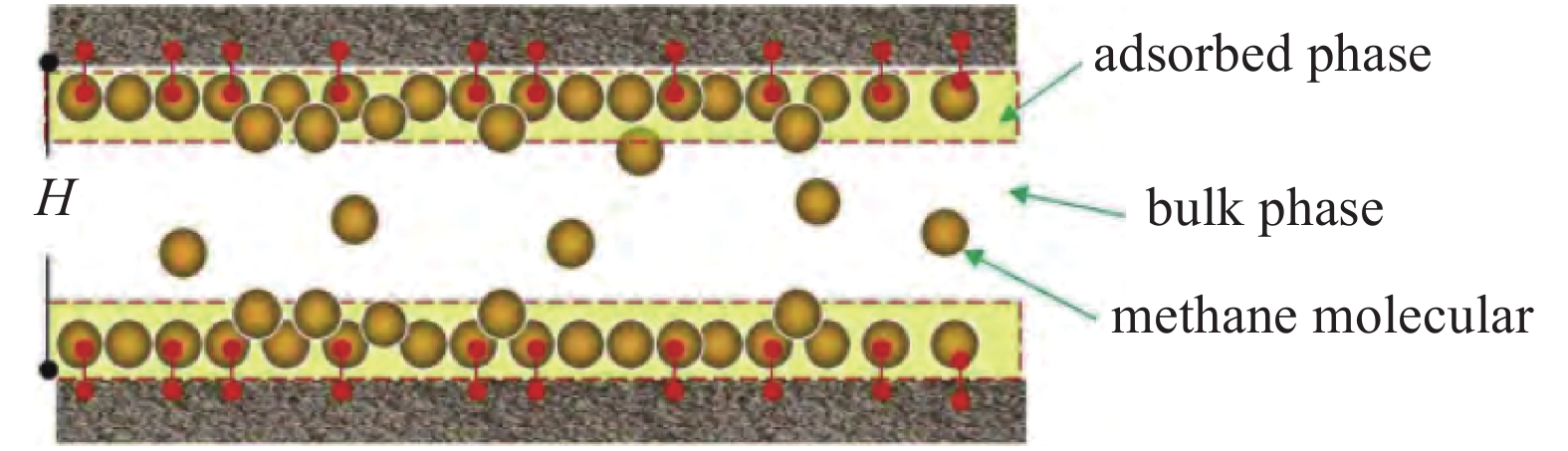
以上主要是页岩本身的物理性质差异带来的吸附能力变化, 除此之外的环境因素如温度压力等也会带来很大影响[32]. 赵丹等[33]提出随着压力的升高, CH4分子的密度随之增大, 也更容易被页岩吸附. 同时密度增大还会导致CH4分子间斥力增大, 甲烷在页岩表面吸附逐渐趋于饱和, 因此压力的变化对吸附量的影响程度随着压力增大而逐步减小. 薛培等[34]分别在30 °C, 45 °C, 60 °C下对页岩做了等温吸附实验, 实验结果表明随着温度的升高, 页岩的吸附性能下降. 同时也提出与前文类似的结论, 影响页岩对CH4吸附能力的因素还有有机质成熟度以及黏土矿物的类型和含量, 成熟度越高的页岩中有机质孔的数量也越多, 比表面积越大, 页岩整体吸附能力越强. 分析五峰组页岩样品发现, 伊利石含量也与吸附CH4量成正相关. 此外, 孔隙中的含水量也是一个影响因素, 李靖等[35]研究了水分含量对CH4吸附的影响, 狭缝孔隙考虑气固界面的CH4吸附模型如图2所示,H为层间距. CH4吸附量可以用Langmuir方程表示为
$$ {n_{{\text{ad-dry}}}} = 2{A_{{\text{slit}}}} K \frac{P}{{P + {P_{\text{L}}}}} $$ (1) 其中nad-dry为甲烷在干燥黏土表面的吸附量, mmol/g; Aslit为单个黏土晶层面积, m2/g; 2Aslit为孔隙比表面积, m2/g; K为单位面积最大吸附量, mmol/m2; PL为兰氏压力, MPa; P为气相压力, MPa.
研究表明水分对孔隙中CH4吸附行为的影响主要表现为: 部分小孔隙被水分阻塞而失去吸附能力; 由于水分子形成水膜, 大孔隙内CH4由气体在固体表面吸附转化为气体在液体表面吸附. 在两者综合作用下, 甲烷吸附能力降低约80% ~ 90%[35-37].
虽然实验方法可以得到真实有效的吸附数据, 但是在进行CH4的吸附实验时通常将页岩样品研磨成粉末, 这就会导致页岩样品的孔隙度、粗糙度以及孔隙之间的连通性发生改变, 所得到的页岩吸附数据与真实页岩气藏的吸附出现偏差. 因此, 在以后的实验过程中如何保持页岩样品的完整性是得到真实页岩储层中页岩气吸附特性的关键, 见图3[38].
由于尺度的限制, 实验研究主要集中在页岩基质的宏观吸附性能上[39], 但是对微观条件下页岩气吸附机理认识不足, 对页岩气运移过程也无法直观. 因此近些年来, 越来越多的研究人员使用分子动力学模拟方法探究页岩纳微米孔中气体的吸附特性[40-41].
通过建立不同的壁面模型, 更改温度压力等条件进行运算是分子动力学模拟方法的主要形式. 王擎等[42]通过建立蒙脱石、高岭石、方解石和生石膏4种矿物质分子模型来进行CH4吸附分子动力学模拟, 比较不同矿物种类对CH4吸附的影响, 在相同的温度压力条件下, 各种矿物类型对CH4气体吸附量的结果为蒙脱石 > 高岭石 > 生石膏 > 方解石. 这与实验结果类似, 说明矿物种类对页岩储层吸附CH4有很大影响. 同时还发现4种矿物中CH4的吸附量随着压力的增大而增大, 直到接近恒定值. CH4吸附量随着温度的增加而减小, 这是因为温度升高增大了CH4分子的动能, 使它不容易被矿物质表面所吸附. Chen等[43]通过分子动力学模拟分别讨论了温度、压力和壁面材料对宏观解吸速度的影响, 也得出了类似的结论: 低压时, 温度越高, 解吸速度越快; 高压时, 温度越高, 解吸速度越慢; 低压时, 不同壁面的解吸速度为石英 > 高岭石 > 石墨, 但在高压条件下时, 解吸速度的大小关系变为石英 > 石墨 > 高岭石. Sui等[44]的研究除了提出吸附量随着压力增加而增加, 随温度升高而减少之外, 还研究了孔径对吸附的影响: 在低压条件下, 微孔中的吸附量大于大孔中的气体吸附量, 在中高压下, 随着孔径的增大, 气体吸附量增大. 以上分子动力学模拟都得到了与实验相似的结论, 即页岩储层中CH4吸附能力与有机质类型、温度、压力、孔隙结构等有关.
但利用分子模拟探讨纳微米尺度下CH4的吸附特性也不尽完善, 比如建立的壁面构型较为单一, 建立狭缝孔、柱状孔以及碳纳米管代替复杂的页岩储层能否被石油工程界认可是一个重要问题. 一方面, 研究人员在建立壁面时应该考虑壁面构型的多样性, 如壁面粗糙度、孔喉与孔隙的迂曲度等问题, 以建立更加真实的页岩孔隙模型; 另一方面, 分子模拟在一定程度上加深了对相关吸附机理的认识, 但模拟得到的结果有时会出现与理论相悖的现象, 如吸附层厚度, 对于这方面的研究需要进一步探讨.
2. CO2/CH4二元混合物竞争吸附特性
页岩储层纳微米孔隙中CO2/CH4二元混合物的竞争吸附特性是探讨注CO2提高页岩气采收率的关键. 陈强等[45]通过实验研究了页岩纳米孔内CH4与CO2混合气体的传输, 色谱监测结果表明, CH4相对于CO2更容易通过页岩, 从而证实了超临界CO2在页岩纳米孔内的传输能力显著弱于CH4. 贾连超等[46]基于核磁共振测试技术对页岩岩芯进行解吸实验, 结果表明注入CO2后吸附态甲烷的解吸效率较自然解吸显著提高, 同时解吸速率从3.01%/h提升至13.69%/h, 这是因为在注入CO2后, 吸附态甲烷的物质的量下降, 转化为游离态, 还说明页岩颗粒及表面对CO2气体的吸附能力大于CH4, 见图4[47].
孙莹等[48]通过实验对比CH4和CO2的吸附能力, 提出在相同条件下, CO2比CH4更易吸附, 因此在页岩储集层中注入CO2可以促进CH4的解吸, 并有利于CO2的地质埋存. 并且在一定范围内, 增加CO2注入速率可以有效提高页岩气的采气速率及采收率. 卓亭妤等[49]向已经达到吸附CH4平衡的鄂尔多斯盆地陆相延长组页岩通入CO2进行实验, 提出在相同的压力下, 随着温度的升高, CH4的解吸速度也随之增大, CO2的封存量则与其密度相关, 密度越大, CO2的封存量也越大. Liu等[50]通过实验研究发现, 注入CO2可使吸附相剩余CH4气体的回收率提高约25%, 在常压下每小时可提高约11%.
多位学者都证明了CO2在与CH4竞争吸附中占据优势, 并且适当改变温度、压力等影响条件可以提高CH4解吸速度[51-56], 但为了定量描述二元混合物在孔隙中竞争吸附行为, 采用选择性系数作为重要参数, 它可以用来评估吸附剂的性能, 选择性系数大于1时, 说明在二元混合物中CO2分子比CH4分子优先吸附于壁面(即吸附能力越强), 选择性系数越大, CO2分子优先级越高[21]. 选择性系数(
$ {S}_{{\text{CO}}_{\text{2}}\text{/}{\text{CH}}_{\text{4}}} $ )定义为$$ {S_{{\text{C}}{{\text{O}}_{\text{2}}}{\text{/C}}{{\text{H}}_{\text{4}}}}} = \frac{{{x_{{\text{C}}{{\text{O}}_{\text{2}}}}}/{x_{{\text{C}}{{\text{H}}_{\text{4}}}}}}}{{{y_{{\text{C}}{{\text{O}}_{\text{2}}}}}/{y_{{\text{C}}{{\text{H}}_{\text{4}}}}}}} $$ (2) 式中
$ {S_{{\text{C}}{{\text{O}}_{\text{2}}}{\text{/C}}{{\text{H}}_{\text{4}}}}} $ 为CO2相对于CH4的选择性,$ {x_{{\text{C}}{{\text{O}}_{\text{2}}}}} $ 和$ {x_{{\text{C}}{{\text{H}}_{\text{4}}}}} $ 分别为吸附相中CO2和CH4的摩尔分数,$ {y_{{\text{C}}{{\text{O}}_{\text{2}}}}} $ 和$ {y_{{\text{C}}{{\text{H}}_{\text{4}}}}} $ 分别为体相(游离相)中CO2和CH4的摩尔分数.相较于实验方法, 许多研究人员选择分子动力学模拟, 更好的从微观角度对纳微米孔隙介质中CO2/CH4二元混合物竞争吸附特性进行研究[57-64], Wang等[65]建立了柱状孔隙模型, 通过分子模拟研究竞争吸附行为, 结果表明, 当压力小于25 MPa时, 温度越低选择系数越大, 压力大于25 MPa时, 温度对选择系数影响逐渐减小; 温度一定时, 选择性系数随着压力的升高而降低; 指出CO2/CH4选择性系数在大埋深时相对较低. Liu等[66]研究了双纳米孔隙中碳氢化合物与CO2的竞争吸附行为, 结果表明, 随着压力的降低, 轻质烃相比较于重质烃更容易被CO2取代, 大孔隙中的碳氢化合物更容易被CO2取代; 在吸附能力方面, 重质烃 > CO2 > 轻质烃; 提出了CO2可以作为回收轻质烃的合适气体, 而对于重质烃则回收效率低下. Zhou等[67]研究了CO2/CH4在高岭石中的竞争吸附行为, 发现CO2存在时, CH4的吸附能力显著降低, 特别是在强吸附层, 如图5所示; 随着温度的升高, 选择性增强; 还发现当含水量增加时, CO2/CH4的吸附量减少并且竞争吸附选择性变小.
CO2/CH4二元混合物在页岩储层纳微米孔隙中的竞争吸附特性是研究注CO2提高页岩气采收率的关键, 目前利用实验方法与分子模拟方法从不同角度探讨了CO2/CH4二元混合物的竞争吸附特性, 但是两种研究方法得到的结果却彼此独立, 并不能关联耦合, 从而无法指导高效采收页岩气. 因此, 在以后的研究中需要建立连接宏观竞争吸附特性与微观竞争吸附的桥梁, 进而指导注CO2提高页岩气采收率技术.
3. CO2/CH4二元混合物驱替特性
页岩储层中气体复杂的贮存形式决定了其开采机理不同, 对于吸附气主要通过注入其他气体对CH4进行置换解吸, 而对于游离气则通过驱替得到. 通过实验方法可以获得气体宏观输运特性, 而分子动力学模拟方法从微观角度来模拟开采过程[68],结合室内实验,建立相应的理论模型描述页岩基质气体输运规律[69], 形成数值模拟技术,这些方法均为提高页岩气开采效率提供了新方法、新思路.
Oldenburg[70]运用数值模拟探讨CO2和CH4的物理性质, 模拟结果表明, 超临界状态下的CO2与CH4在密度、黏度和压缩性方面差异显著, 因此, 利用超临界CO2作为驱替CH4的驱替气体是完全可行的. Hughes等[71]通过实验证明了超临界CO2在CH4中的分散性小于0.0001 m, 并提出两者的流度比小于1, 因此黏性指进也不会发生[72]. 其分散性描述如下
$$ \frac{{\partial C}}{{\partial {t}}} + u(r)\frac{{\partial C}}{{\partial x}} = D\left(\frac{{{\partial ^2}C}}{{\partial {x^2}}} + \frac{1}{r}\frac{\partial }{{\partial r}}r\frac{{\partial c}}{{\partial r}}\right) $$ (3) 其中C分别为示踪剂浓度, u(r)为管内径向速度, D为分子扩散系数, t是时间, r为分子到管中心的径向距离, x为沿管的距离.
Sidiq和Amin[73]在研究超临界CO2驱替CH4分散性的过程中, 成功捕获了超临界CO2与CH4的非混相界面. 此外还有许多学者的研究都证明了超临界CO2驱替CH4有别于普通气体的驱替过程, 在两者之间可能存在一个过渡带, 这为利用超临界CO2驱替CH4提供了理论支持.
在前人的基础上, 许多研究人员利用实验与数值模拟对一维CO2驱替CH4过程中的成分分布进行了探讨. Sidiq和Amin[74]利用实验研究了不同孔隙压力对CO2驱替CH4的影响, 结果表明施加最高孔隙压力(40.68 MPa)可以提高约40%的回收率. 同时在实验过程中还发现, 当两种气相在多孔介质中相遇时, 它们的界面是从一种纯相到另一种纯相的混溶区, 即在两种纯相之间存在着过渡带. 通过对实验数据进行最佳拟合, 得到气−气相相对渗透率模型如下
$$ {K_{r{\text{c}}{{\text{o}}_2}}} = {{a}} {{S^b_{{\text{C}}{{\text{O}}_{\text{2}}}}}} $$ (4) $$ {K_{r{{\text{c}}_1}}} = {K^{{c}}_{r{\text{c}}{{\text{o}}_{\text{2}}}}} [(1 - {S_{{\text{C}}{{\text{O}}_{\text{2}}}}}) {f_{\text{c}}}] $$ (5) 其中
${K_{r{\text{c}}{{\text{o}}_{\text{2}}}}}$ ,${K_{r{{\text{c}}_1}}}$ 分别为CO2与CH4的相对渗透率,${S_{{\text{C}}{{\text{O}}_{\text{2}}}}}$ 为超临界CO2的饱和度, fc为原始气体中的甲烷浓度分数, 方程中的变量a, b, c则通过方程(4)和方程(5)对实验数据的拟合得到.许多研究人员也得到了类似的结论[75-82], 其中Shi等[81]进行了致密/低渗透储层下CO2驱替CH4的实验, 结果表明注入超临界CO2时, CO2不会广泛地与天然气混合, 而是部分地与天然气混合. 同时由于超临界CO2的密度大于天然气, 因此CO2倾向于沉降于储层底部, 形成受倾角约束的驱替锋面.
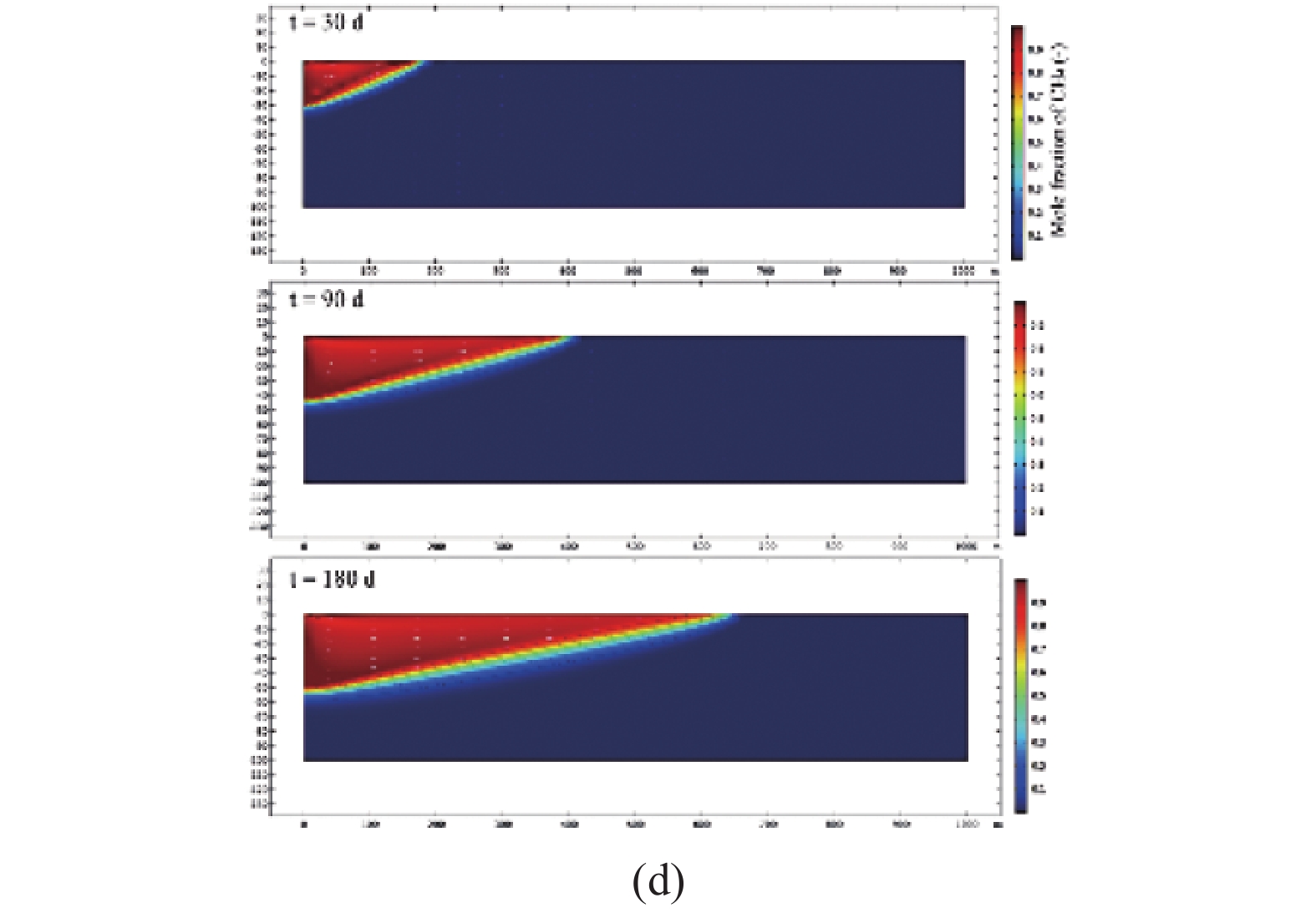
Ma等[82]采用二维模型运用流体力学进行模拟, 得到不同注入时间下混合区的分布情况如图6所示. 模拟结果表明随着CH4的不断注入, 二氧化碳在储层边界处被驱替和压缩, 但混合程度明显增加. 同时混相界面的混合程度和倾角随储层厚度的增加而减小. 以上学者的研究均证明了超临界CO2驱替CH4的过程存在着一个过渡带能够起到类似活塞驱替的效果.
![]() 图 6 (a)注气后22 m厚储层混合区域的分布; (b)不考虑重力影响, 注气后22 m厚储层混合区域的分布; (c)注气后50 m厚储层混合区分布; (d)注气后100 m厚储层混合区分布[82]Figure 6. (a) The distribution of the 22 m thick reservoir mixing zone after gas injection. (b) The distribution of the mixing zone of 22 m thick reservoir after gas injection without considering the influence of gravity. (c) The distribution of the 50 m thick reservoir mixing zone after gas injection. (d) The distribution of the 100 m thick reservoir mixing zone after gas injection[82]
图 6 (a)注气后22 m厚储层混合区域的分布; (b)不考虑重力影响, 注气后22 m厚储层混合区域的分布; (c)注气后50 m厚储层混合区分布; (d)注气后100 m厚储层混合区分布[82]Figure 6. (a) The distribution of the 22 m thick reservoir mixing zone after gas injection. (b) The distribution of the mixing zone of 22 m thick reservoir after gas injection without considering the influence of gravity. (c) The distribution of the 50 m thick reservoir mixing zone after gas injection. (d) The distribution of the 100 m thick reservoir mixing zone after gas injection[82]目前对于油气藏的注采方式多采用井网形式, 因此研究井网条件下CO2/CH4驱替是很有必要的, 能够为后期CH4的开采提供理论指导. Patel等[83]通过数值模拟建立了CO2驱替CH4的5点井网模型, 研究1/4单元中驱替锋面的移动情况如图7所示.
![]() 图 7 (a)三维模拟结果的平面. IW和PW分别表示注入井和生产井的位置; (b)超临界层状油藏模拟基本情况的成分分布的时间演化[83]Figure 7. (a) 3D simulation results of the plane. IW and PW represent the location of the injection and production well. (b) Temporal evolution of component distribution in a supercritical layered reservoir simulation base case[83]
图 7 (a)三维模拟结果的平面. IW和PW分别表示注入井和生产井的位置; (b)超临界层状油藏模拟基本情况的成分分布的时间演化[83]Figure 7. (a) 3D simulation results of the plane. IW and PW represent the location of the injection and production well. (b) Temporal evolution of component distribution in a supercritical layered reservoir simulation base case[83]图7(a)为三维油藏模拟的平面. 虚线表示渗透率不同的岩层, IW和PW分别表示注入井和生产井的位置. 图7(b)为超临界层状油藏模拟基本情况的成分分布的时间演化. 箭头表示局部流动方向, 长度与流体速度的对数成正比. 从图中可以看出驱替锋面较为狭窄, 这是因为超临界CO2与CH4的性质差异显著, 所以导致超临界CO2在CH4中的扩散系数较小[71]. 同时Patel等[83]通过模拟得到一个关键结果, 将注入速度提高10倍, CO2的突破时间仅减少6.7 ~ 8.2倍, 同时会使得CH4采收率提高.
Song等[84]根据纳米多孔介质储层中页岩气的运移机理, 建立了一种新的多尺度气体运动方程和页岩气运移数学模型. 提出在压降压力、裂缝半长、裂缝导流能力、扩散系数和裂缝间距之间进行优化配置, 实现页岩气高效产能.
Liu等[85]建立了注入井的位置、数量以及方式3种数值模型, 模拟结果表明重力对储层中天然气的驱替作用是积极的. 因此, 为了获得更好的CH4采收率和更大的CO2储存能力, 最好选择储层顶部作为射孔位置注入CO2. Biagi等[86]对5点井网注入方式下CO2注入速率进行了优化, 模拟结果表明最优注入速率为0.294 kg/s, 最优注入速率可以显著提高CH4产量, 提高采收率, 缩短关井时间. 综上所述, 注入井的位置、数量以及注入速率均对突破时间和产量有影响, 因此从三方面均可进行优化考虑, 从而得到最佳采收率[87-88].
4. 讨论及关键问题分析
许多研究人员利用不同的手段从不同的尺度探究气体在页岩储层纳微米孔隙中的吸附特性, 基于前人研究工作, 本文对气体在页岩储层中吸附特性的基础研究及关键问题进行讨论分析并提出如下展望.
(1) 利用实验方法研究CH4的吸附特性可以认为是流动状态下的实验结果, 而利用分子模拟方法的研究主要集中在非流动状态下; 气体在流动状态下与非流动状下表现出的流体特性是完全不同的, 如何使分子模拟方法更加接近真实页岩储层条件需要进一步研究; 另一方面, 页岩气的吸附层厚度, 页岩有效孔隙度和成熟度等, 与准确评价页岩气储量相关的参数是未来研究页岩气吸附特性的重要方向. 此外, 在实验过程中如何采用真实岩心并实现流动状态下吸附特性的研究更能逼近真实页岩储层.
(2) 在探讨页岩储层中CO2/CH4二元混合物竞争吸附特性时, 如何定量描述不同因素影响下(含水率、页岩成熟度、复杂页岩气成分等)竞争吸附效率是提高页岩气采收率的关键; 另一方面, 通过基础理论建立数学模型探讨CO2/CH4二元混合物竞争吸附特性对于注CO2提高页岩气采收率机理分析至关重要, 但是相关研究较少, 在以后的研究中可以进一步考虑分析.
(3) 利用CO2驱替CH4不同于一般气体的混合, 在两者之间存在着过渡带能够起到类似活塞驱替的效果, 这一结论对理解CO2驱替页岩气有重要意义, 但对于两者之间的过渡带更多的是通过实验测定出口处气体成分来确定, 而关于过渡带在移动过程中的特性仍不明朗. 同时对影响突破时间和累计产量的因素, 如注入井的位置、数量以及注入时间等缺乏系统性比较. 因此如何从实验角度实现可视化的驱替过程仍然是有待研究的重要课题.
(4) 在注CO2提高页岩气采收率的相关研究中, CH4的吸附特性、CO2/CH4二元混合物竞争吸附特性以及驱替特性的研究涉及多个尺度, 包括从纳微米级孔隙到天然裂缝, 不同尺度下的气体吸附机理对气藏的开采至关重要. 如何将多尺度吸附及流动机理耦合, 建立注CO2提高页岩气采收率产能模型, 从而准确评价和高效开采页岩气藏是亟待解决的关键问题.
5. 总结
页岩气贮存形式复杂并且页岩储层孔隙结构在纳微米级尺度, 这导致页岩气的开采难度大. CH4在页岩储层中表现为物理吸附, 有机质特征(丰度、成熟度、类型)、纳米孔隙结构、无机矿物组成、温度和压力、含水率对页岩的CH4吸附能力均有一定程度的影响. 在相同条件下, CO2比CH4更易被页岩储层吸附, 在页岩储层中注入CO2可以促进CH4的解吸, 并有利于CO2的地质埋存. 利用CO2开采页岩气不仅能够提高页岩气采收率, 并且对CO2进行地质封存, 有助于实现页岩气开采过程的碳中和. 开采方案的部署可采用井网形式的注采方式, 可以通过调整注入井的位置、数量以及CO2注入速率对开采方案进行优化.
-
图 6 (a)注气后22 m厚储层混合区域的分布; (b)不考虑重力影响, 注气后22 m厚储层混合区域的分布; (c)注气后50 m厚储层混合区分布; (d)注气后100 m厚储层混合区分布[82]
Figure 6. (a) The distribution of the 22 m thick reservoir mixing zone after gas injection. (b) The distribution of the mixing zone of 22 m thick reservoir after gas injection without considering the influence of gravity. (c) The distribution of the 50 m thick reservoir mixing zone after gas injection. (d) The distribution of the 100 m thick reservoir mixing zone after gas injection[82]
图 7 (a)三维模拟结果的平面. IW和PW分别表示注入井和生产井的位置; (b)超临界层状油藏模拟基本情况的成分分布的时间演化[83]
Figure 7. (a) 3D simulation results of the plane. IW and PW represent the location of the injection and production well. (b) Temporal evolution of component distribution in a supercritical layered reservoir simulation base case[83]
-
[1] Wang M, Ronald WT, Song G, et al. Geochemical and geological characteristics of the Es3 L lacustrine shale in the Bonan sag, Bohai Bay Basin, China. International Journal of Coal Geology, 2015, 138: 16-29 doi: 10.1016/j.coal.2014.12.007
[2] Cardott BJ. Thermal maturity of Woodford Shale gas and oil plays, Oklahoma, USA. International Journal of Coal Geology, 2012, 103: 109-119 doi: 10.1016/j.coal.2012.06.004
[3] 王晓琦, 翟增强, 金旭等. 页岩气及其吸附与扩散的研究进展. 化工学报, 2015, 66(8): 2838-2845 (Wang Xiaoqi, Zhai Zengqiang, Jin Xu, et al. Progress in adsorption and diffusion of shale gas. Journal of Chemical Industry and Engineering, 2015, 66(8): 2838-2845 (in Chinese) [4] Zeng B, Duan H, Bai Y, et al. Forecasting the output of shale gas in China using an unbiased grey model and weakening buffer operator. Energy, 2018, 151: 238-249 doi: 10.1016/j.energy.2018.03.045
[5] Li A, Han W, Fang Q, et al. Experimental investigation of methane adsorption and desorption in water-bearing shale. Capillarity, 2002, 3(3): 45-55
[6] Curtis JB. Fractured shale-gas systems. AAPG Bulletin, 2002, 86(11): 1921-1938
[7] Ji K, Guo S, Hou B. A logging calculation method for shale adsorbed gas content and its application. Journal of Petroleum Science and Engineering, 2017, 150: 250-256 doi: 10.1016/j.petrol.2016.12.008
[8] Li P, Jiang Z, Zheng M, et al. Estimation of shale gas adsorption capacity of the Longmaxi formation in the upper Yangtze Platform, China. Journal of Natural Gas Science and Engineering, 2016, 34: 1034-1043 doi: 10.1016/j.jngse.2016.07.052
[9] Feng Q, Xu S, Xing X, et al. Advances and challenges in shale oil development: A critical review. Advances in Geo-Energy Resarch, 2020, 4(4): 406-418 doi: 10.46690/ager.2020.04.06
[10] Gregg SJ, Sing KSW. Adsorption surface area and porosity. Academic Press, 1982, 94(2): 597-598
[11] Wang J, Song H, Rasouli V, et al. An integrated approach for gas-water relative permeability determination in nanoscale porous media. Journal of Petroleum Science and Engineering, 2019, 173: 237-245 doi: 10.1016/j.petrol.2018.10.017
[12] Gao Z, Fan Y, Xuan Q, et al. A review of shale pore structure evolution characteristics with increasing thermal maturities. Advances in Geo-Energy Research, 2020, 4(3): 247-259 doi: 10.46690/ager.2020.03.03
[13] Zhang Q, Liu R, Pang Z, et al. Chracterization of microscopic pore structures in lower silurian black shale (S1l), southeastern Chongqing, China. Marine and Petroleum Geology, 2016, 71: 250-259 doi: 10.1016/j.marpetgeo.2015.12.015
[14] Chen H, Carter KE. Water usage for natural gas production through hydraulic fracturing in the United States from 2008 to 2014. Journal of Environmental Management, 2016, 170: 152-159
[15] Clarkson CR, Haghshenas B, Ghanizadeh A, et al. Nanopores to megafractures: current challenges and methods for shale gas reservoir and hydraulic fracture characterization. Journal of Natural Gas Science and Engineering, 2016: 612-657
[16] Mohamadi A, Behnia M, Alneasan M. Comparison of the classical and fracture mechanics approaches to determine in situ stress/hydrofracturing method. Bulletin of Engineering Geology and the Environment, 2021, 80: 3833-3851
[17] Zhang L, Wang Y, Miao X, et al. Geochemistry in geologic CO2 utilization and storage: A brief review. Advances in Geo-Energy Resarch, 2019, 3(3): 304-313 doi: 10.26804/ager.2019.03.08
[18] Makaremi M, Jordan KD, Guthrie GD, et al. Multiphase Monte Carlo and molecular dynamics simulations of water and CO2 intercalation in montmorillonite and beidellite. The Journal of Physical Chemistry C, 2015, 119(27): 15112-15124 doi: 10.1021/acs.jpcc.5b01754
[19] Sun H, Zhao H, Qi N, et al. Molecular insight into the micro-behaviors of CH4 and CO2 in montmorillonite slit-nanopores. Molecular simulation, 2017, 43(13-16): 1004-1011 doi: 10.1080/08927022.2017.1328553
[20] 尹帅, 单钰铭, 郑莲慧等. 页岩气等温吸附量及等量吸附热研究. 科学技术与工程, 2013, 13(29): 8572-8578 (Yin Shuai, Shan Yuming, Zheng Lianhui, et al. Research of shale gas isothemal adsorption quantity and equal amount adsorption heat. Science Technology and Engineering, 2013, 13(29): 8572-8578 (in Chinese) doi: 10.3969/j.issn.1671-1815.2013.29.005 [21] 隋宏光, 姚军. CO2/CH4在干酪根中竞争吸附规律的分子模拟. 中国石油大学学报, 2016, 40(2): 147-154 (Sui Hongguang, Yao Jun. Molecular simulation of CO2/CH4 competitive adsorption in kerogen. Journal of China University of Petroleum, 2016, 40(2): 147-154 (in Chinese) [22] Chen L, Liu K, Jiang S, et al. Effect of adsorbed phase density on the correction of methane excess adsorption to absolute adsorption in shale. Chemical Engineering Journal, 2021, 420: 127678 doi: 10.1016/j.cej.2020.127678
[23] Wang T, Tian S, Liu Q, et al. Pore structure characterization and its effect on methane adsorption in shale kerogen. Petroleum Science, 2021, 18(2): 565-578 doi: 10.1007/s12182-020-00528-9
[24] Onawole AT, Nasser MS, Hussein IA, et al. Theoretical studies of methane adsorption on Silica-Kaolinite interface for shale reservoir application. Applied Surface Science, 2021, 546: 149164 doi: 10.1016/j.apsusc.2021.149164
[25] Tian W, Liu H. Insight into the adsorption of methane on gas shales and the induced shale swelling. ACS Omega, 2020, 5(49): 31508-31517 doi: 10.1021/acsomega.0c02980
[26] Kong S, Huang X, Li K, et al. Adsorption/desorption isotherms of CH4 and C2H6 on typical shale samples. Fuel, 2019, 255: 115632
[27] 郭春礼, 杨爽, 王安东等. 修武盆地下寒武统海相页岩储层及CH4吸附特征. 天然气地球科学, 2021, 32(4): 598-610 (Guo Chunli, Yang Shuang, Wang Andong, et al. Study on the lower cambrian marine shale reservoir and methane adsorption characteristics in Xiuwu Basin. Natural Gas Geoscience, 2021, 32(4): 598-610 (in Chinese) [28] Zhou S, Ning Y, Wang H, et al. Investigation of methane adsorption mechanism on Longmaxi shale by combining the micropore filling and monolayer coverage theories. Advances in Geo-Energy Resarch, 2018, 2(3): 269-281 doi: 10.26804/ager.2018.03.05
[29] 沈伟军, 李熙喆, 鲁晓兵等. 基于等温吸附的页岩水分传输特征研究. 力学学报, 2019, 51(3): 932-939 (Shen Weijun, Li Xizhe, Lu Xiaobing, et al. Study on moisture transport characteristics of shale based on isothermal adsorption. Chinese Journal of Theoretical and Applied Mechanics, 2019, 51(3): 932-939 (in Chinese) [30] 陈磊, 姜振学, 温暖等. 页岩纳米孔隙分形特征及其对甲烷吸附性能的影响. 科学技术与工程, 2017, 17(2): 31-39 (Chen Lei, Jiang Zhenxue, Wen Nuan, et al. Fractal characteristics of nanopores and their effect on methane adsorption capacity in shales. Science Technology and Engineering, 2017, 17(2): 31-39 (in Chinese) doi: 10.3969/j.issn.1671-1815.2017.02.006 [31] 朱汉卿, 贾爱林, 位云生等. 蜀南地区富有机质页岩孔隙结构及超临界甲烷吸附能力. 石油学报, 2018, 39(4): 391-401 (Zhu Hanqing, Jia Ailin, Wei Yunsheng, et al. Pore structure and supercritical methane sorption capacity of organic-rich shales in southern sichuan basin. Acta Petrolei Sinica, 2018, 39(4): 391-401 (in Chinese) [32] Mu Z, Ning Z, Ren C. Methane adsorption on shales and application of temperature-related composite models based on dual adsorption modes. Journal of Petroleum Science and Engineering, 2022, 208: 109379 doi: 10.1016/j.petrol.2021.109379
[33] 赵丹, 蔡长宏, 安珏东等. 页岩中基于孔隙度和有机碳含量的甲烷吸附量计算. 石油与天然气化工, 2018, 23(2): 31-38 (Zhao Dan, Cai Changhong, An Yudong, et al. Calculation of methane adsorption in shale based on porosity and organic carbon content. Chemical Engineering of Oil & Gas, 2018, 23(2): 31-38 (in Chinese) doi: 10.3969/j.issn.1007-3426.2018.02.006 [34] 薛培, 高栋臣, 孙建博等. 鄂尔多斯盆地延长组页岩吸附热力学特征. 煤田地质与勘探, 2018, 46(4): 22-27 (Xue Pei, Gao Dongchen, Sun Jianbo, et al. Adsorption thermodynamic property of yanchang formation shale in ordos basin. Coal Geology & Exploration, 2018, 46(4): 22-27 (in Chinese) doi: 10.3969/j.issn.1001-1986.2018.04.004 [35] 李靖, 李相方, 王香增等. 页岩黏土孔隙含水饱和度分布及其对甲烷吸附的影响. 力学学报, 2016, 48(5): 1217-1228 (Li Jing, Li Xiangfang, Wang Xiangzeng, et al. Effect of water distribution on methane adsorption capacitu in shale clay. Chinese Journal of Theoretical and Applied Mechanics, 2016, 48(5): 1217-1228 (in Chinese) [36] Bai J, Kang Y, Chen M, et al. Impact of water film on methane surface diffusion in gas shale organic nanopores. Journal of Petroleum Science and Engineering, 2021, 196: 108045 doi: 10.1016/j.petrol.2020.108045
[37] Zhao P, Xie L, He B, et al. Anisotropic permeability influencing the performance of free CH4 and free CO2 during the process of CO2 sequestration and enhanced gas recovery (CS-EGR) from shale. ACS Sustainable Chemistry & Engineering, 2021, 9(2): 914-926
[38] Xu LW, Liu LF, Jiang ZX, et al. Methane adsorption in the low-middle-matured Neoproterozoic Xiamaling marine shale in Zhangjiakou, Hebei. Australian Journal of Earth Sciences, 2018, 65(5): 691-710 doi: 10.1080/08120099.2018.1455741
[39] Gensterblum Y, Busch A, Krooss BM. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel, 2014, 115: 581-588 doi: 10.1016/j.fuel.2013.07.014
[40] Xiong J, Liu X, Liang L, et al. Adsorption of methane in organic-rich shale nanopores: ans experimental and molecular simulation study. Fuel, 2017, 200: 299-315 doi: 10.1016/j.fuel.2017.03.083
[41] Chen S, Zhang C, Li X, et al. Simulation of methane adsorption in diverse organic pores in shale reservoirs with multi-period geological evolution. International Journal of Coal Science & Technology, 2021, 8: 844-855
[42] 王擎, 李础安, 潘朔等. CH4和CO2在油页岩中矿物质结构内部吸附的分子模拟. 燃料化学学报, 2017, 45(11): 1310-1316 (Wang Qing, Li Chuan, Pan Shuo, et al. A molecular simulation study on the adsorption of CH4 and CO2 on the mineral substances in oil shale. Journal of Fuel Chemistry and Technology, 2017, 45(11): 1310-1316 (in Chinese) doi: 10.3969/j.issn.0253-2409.2017.11.005 [43] Chen L, Huang D, Wang S, et al. A study on dynamic desorption process of methane in slits. Energy, 2019, 175: 1174-1180 doi: 10.1016/j.energy.2019.03.148
[44] Sui H, Yao J, Zhang L. Molecular simulation of shale gas adsorption and diffusion in clay nanopores. Computation, 2015, 3(4): 687-700 doi: 10.3390/computation3040687
[45] 陈强, 孙雷, 潘毅等. 页岩纳米孔内超临界CO2、CH4传输行为实验研究. 西南石油大学学报(自然科学版), 2018, 40(5): 154-162 (Chen Qiang, Sun Lei, Pan Yi, et al. Experimental study on the transmission behaviors of supercritical CO2 and CH4 in shale nanapores. Journal of Southwest Petroleum University (Science &Technology Edition) , 2018, 40(5): 154-162 (in Chinese) [46] 贾连超, 刘鹏飞, 袁丹等. 注CO2提高页岩吸附气采收率实验——以鄂尔多斯盆地延长组长7页岩气为例. 大庆石油地质与开发, 2021, 40(2): 153-159 (Jia Lianchao, Liu Pengfei, Yuan Dan, et al. Experiment of enhancing the recovery of the shale adsorbed gas by CO2 injection: taking yanchang-formation chang-7 shale gas in ordos basin as an example. Petroleum Geology &Oilfield Development in Daqing, 2021, 40(2): 153-159 (in Chinese) [47] 杨飞, 岳长涛, 李术元等. 四川盆地志留系页岩CH4和CO2吸附特征. 化工学报, 2017, 68(10): 3851-3859 (Yang Fei, Yue Changtao, Li Shuyuan, et al. Adsorption characteristics of CH4 and CO2 on silurian shale in sichuan basin. Journal of Chemical Industry and Engineering, 2017, 68(10): 3851-3859 (in Chinese) [48] 孙莹, 孙仁远, 刘晓强等. 基于竞争吸附的页岩气藏提高采收率机理. 新疆石油地质, 2021, 42(2): 224-231 (Sun Ying, Sun Renyuan, Liu Xiaoqiang, et al. Mechanism of enhanced gas recovery in shale gas reservoirs based on competitive adsorption. Xinjiang Petroleum Geology, 2021, 42(2): 224-231 (in Chinese) [49] 卓亭妤, 都喜东, 周敏琪. 不同状态CO2驱替页岩吸附CH4的动态特性研究. 中国科技论文, 2020, 15(5): 509-514 Zhuo Tingyu, Du Xidong, Zhou Minqi, Study on dynamic characteristics about the displacement of adsorbed CH4 on shale by injecting different states of CO2. China Science Paper, 2020, 15(5): 509-514 (in Chinese)
[50] Liu J, Yao Y, Liu D, et al. Experimental evaluation of CO2 enhanced recovery of adsorbed-gas from shale. International journal of coal geology, 2017, 179: 211-218 doi: 10.1016/j.coal.2017.06.006
[51] Luo X, Wang S, Wang Z, et al. Adsorption of methane, carbon dioxide and their binary mixtures on Jurassic shale from the Qaidam Basin in China. International Journal of Coal Geology, 2015, 150-151: 210-223 doi: 10.1016/j.coal.2015.09.004
[52] Lee H, Kim H, Shi Y, et al. Competitive adsorption of CO2/CH4 mixture on dry and wet coal from subcritical to supercritical conditions. Chemical Engineering Journal, 2013, 230: 93-101 doi: 10.1016/j.cej.2013.06.036
[53] Kang G, Zhang B, Kang T, et al. Effect of Pressure and temperature on CO2/CH4 competitive adsorption on kaolinite by Monte Carlo simulations. Materials, 2020, 13(12): 2851 doi: 10.3390/ma13122851
[54] Du X, Gu M, Hou Z, et al. Experimental study on the kinetics of adsorption of CO2 and CH4 in Gas-Bearing shale reservoirs. Energy & Fuels, 2019, 33(12): 12587-12600
[55] Taraba B. Flow calorimetric insight to competitive sorption of carbon dioxide and methane on coal. Thermochimica Acta, 2011, 523(1-2): 250-252 doi: 10.1016/j.tca.2011.05.007
[56] Liu J, Xie H, Wang Q, et al. Influence of pore structure on shale gas recovery with CO2 sequestration: Insight into molecular mechanisms. Energy & Fuels, 2020, 34(2): 1240-1250
[57] Yang N, Liu S, Yang X. Molecular simulation of preferential adsorption of CO2 over CH4 in namontmorillonite clay material. Applied Surface Science, 2015, 356: 1262-1271 doi: 10.1016/j.apsusc.2015.08.101
[58] Wang T, Tian S, Li G, et al. Selective adsorption of supercritical carbon dioxide and methane binary mixture in shale kerogen nanopores. Journal of Natural Gas Science and Engineering, 2018, 50: 181-188 doi: 10.1016/j.jngse.2017.12.002
[59] Chong L, Myshakin EM. The effect of residual water content on preferential adsorption in carbon dioxide – methane – illite clay minerals: A molecular simulation study. Fluid Phase Equilibria, 2020, 504: 112333 doi: 10.1016/j.fluid.2019.112333
[60] Chen C, Hu W, Sun J, et al. CH4 adsorption and diffusion in shale pores from molecular simulation and a model for CH4 adsorption in shale matrix. International Journal of Heat and Mass Transfer, 2019, 141: 367-378 doi: 10.1016/j.ijheatmasstransfer.2019.06.087
[61] Zhai Z, Wang X, Jin X, et al. Adsorption and diffusion of shale gas reservoirs in modeled clay minerals at different geological depths. Energy & Fuels, 2014, 28(12): 7467-7473
[62] Chen G, Lu S, Liu K, et al. GCMC simulations on the adsorption mechanisms of CH4 and CO2 in K-illite and their implications for shale gas exploration and development. Fuel, 2018, 224: 521-528 doi: 10.1016/j.fuel.2018.03.061
[63] Sharma A, Namsani S, Singh JK. Molecular simulation of shale gas adsorption and diffusion in inorganic nanopores. Molecular Simulation, 2015, 41(5-6): 414-422 doi: 10.1080/08927022.2014.968850
[64] Li Y, Hu Z, Liu X, et al. Pressure-dependent equilibrium molecular simulation of shale gas and its distribution and motion characteristics in organic-rich nano-slit. Fuel, 2019, 237: 1040-1049 doi: 10.1016/j.fuel.2018.10.050
[65] Wang X, Zhai Z, Jin X, et al. Molecular simulation of CO2/CH4 competitive adsorption in organic matter pores in shale under certain geological conditions. Petroleum Exploration and Development Online, 2016, 43(5): 841-848 doi: 10.1016/S1876-3804(16)30100-8
[66] Liu Y, Ma X, Li HA, et al. Competitive adsorption behavior of hydrocarbon(s)/CO2 mixtures in a double-nanopore system using molecular simulations. Fuel, 2019, 252: 612-621 doi: 10.1016/j.fuel.2019.04.123
[67] Zhou W, Wang H, Yan Y, et al. Adsorption mechanism of CO2/CH4 in Kaolinite clay: insight from molecular simulation. Energy & Fuels, 2019, 33(7): 6542-6551
[68] 蔡建超, 夏宇轩, 徐赛等. 含水合物沉积物多相渗流特性研究进展. 力学学报, 2020, 52(1): 208-223 (Cai Jianchao, Xia Yuxuan, Xu Sai, et al. Advaneces in multiphase seepage characteristics of natural gas hydrate sediments. Chinese Journal of Theoretical and Applied Mechanics, 2020, 52(1): 208-223 (in Chinese) [69] 宋洪庆, 刘启鹏, 于明旭等. 页岩气渗流特征及压裂井产能. 北京科技大学学报, 2014(2): 139-144 (Song Hongqing, Liu Qipeng, Yu Mingxu, et al. Characteristics of gas flow and productivity of fractured wells in shale gas sediments. Journal of University of Science and Technology Beijing, 2014(2): 139-144 (in Chinese) [70] Oldenburg CM. Carbon dioxide as cushion gas for natural gas storage. Energy & Fuels, 2003, 17(1): 240-246
[71] Hughes TJ, Honari A, Graham BF, et al. CO2 sequestration for enhanced gas recovery: new measurements of supercritical CO2–CH4 dispersion in porous media and a review of recent research. International Journal of Greenhouse Gas Control, 2012, 9: 457-468 doi: 10.1016/j.ijggc.2012.05.011
[72] Oldenburg CM, Pruess K, Benson SM. Process modeling of CO2 injection into natural gas reservoirs for carbon sequestration and enhanced gas recovery. Energy & Fuels, 2001, 15(2): 293-298
[73] Sidiq H, Amin R. Mathematical model for calculating the dispersion coefficient of super critical CO2 from the results of laboratory experiments on enhanced gas recovery. Journal of Natural Gas Science and Engineering, 2009, 1(6): 177-182 doi: 10.1016/j.jngse.2009.11.001
[74] Sidiq H, Amin R. The Impact of pore pressure on CO2-methane displacement. Petroleum Science and Technology, 2012, 30(24): 2531-2542 doi: 10.1080/10916466.2010.516300
[75] Ding J, Yan C, He Y, et al. Supercritical CO2 sequestration and enhanced gas recovery in tight gas reservoirs: Feasibility and factors that influence efficiency. International Journal of Greenhouse Gas Control, 2021, 105: 103234 doi: 10.1016/j.ijggc.2020.103234
[76] Liu S, Sun B, Xu J, et al. Study on competitive adsorption and displacing properties of CO2 enhanced shale gas recovery: advances and challenges. Geofluids, 2020, 2020: 1-15
[77] Luo E, Hu Y, Wang J, et al. The effect of impurity on miscible CO2 displacement mechanism. Oil & Gas Science and Technology – Revue d’IFP Energies Nouvelles, 2019, 74: 86
[78] Chen R, Zhang G, Yi C. Research progress and prospects of CO2 enhanced shale gas recovery and geologic sequestration. E3S Web of Conferences, 2018, 53: 4002 doi: 10.1051/e3sconf/20185304002
[79] Huo P, Zhang D, Yang Z, et al. CO2 geological sequestration: Displacement behavior of shale gas methane by carbon dioxide injection. International Journal of Greenhouse Gas Control, 2017, 66: 48-59 doi: 10.1016/j.ijggc.2017.09.001
[80] Hou P, Ju Y, Gao F, et al. Simulation and visualization of the displacement between CO2 and formation fluids at pore-scale levels and its application to the recovery of shale gas. International Journal of Coal Science & Technology, 2016, 3(4): 351-369
[81] Shi Y, Jia Y, Pan W, et al. Potential evaluation on CO2-EGR in tight and low-permeability reservoirs. Natural Gas Industry B, 2017, 4(4): 311-318 doi: 10.1016/j.ngib.2017.08.013
[82] Ma J, Li Q, Kempka T, et al. Hydromechanical response and impact of gas mixing behavior in subsurface CH4 storage with CO2-based cushion gas. Energy & Fuels, 2019, 33(7): 6527-6541
[83] Patel MJ, May EF, Johns ML. High-fidelity reservoir simulations of enhanced gas recovery with supercritical CO2. Energy, 2016, 111: 548-559 doi: 10.1016/j.energy.2016.04.120
[84] Song H, Yu M, Zhu W, et al. Numerical investigation of gas flow rate in shale gas reservoirs with nanoporous media. International Journal of Heat and Mass Transfer, 2015, 80: 626-635 doi: 10.1016/j.ijheatmasstransfer.2014.09.039
[85] Liu S, Agarwal R, Sun B, et al. Numerical simulation and optimization of injection rates and wells placement for carbon dioxide enhanced gas recovery using a genetic algorithm. Journal of Cleaner Production, 2021, 280: 2-21
[86] Biagi J, Agarwal R, Zhang Z. Simulation and optimization of enhanced gas recovery utilizing CO2. Energy, 2016, 94: 78-86 doi: 10.1016/j.energy.2015.10.115
[87] Luo C, Zhang D, Lun Z, et al. Displacement behaviors of adsorbed coalbed methane on coals by injection of SO2/CO2 binary mixture. Fuel, 2019, 247: 356-367 doi: 10.1016/j.fuel.2019.03.057
[88] Liu J, Xie L, He B, et al. Performance of free gases during the recovery enhancement of shale gas by CO2 injection: a case study on the depleted Wufeng–Longmaxi shale in northeastern Sichuan Basin, China. Petroleum Science, 2021, 18(2): 530-545 doi: 10.1007/s12182-020-00533-y




 下载:
下载: